Make sure you get the green bottle

DISCOVER ALPHAGAN® P 0.1%
What is glaucoma?
Glaucoma is a disease that can damage the eye’s optic nerve. It usually develops when fluid pressure inside the eye (or eye pressure) slowly rises, causing high eye pressure, or ocular hypertension. Glaucoma is a leading cause of blindness.
What is ALPHAGAN® P 0.1%?
ALPHAGAN® P 0.1% is a prescription eye drop approved to lower eye pressure (IOP) in patients with open-angle glaucoma or high eye pressure (ocular hypertension).
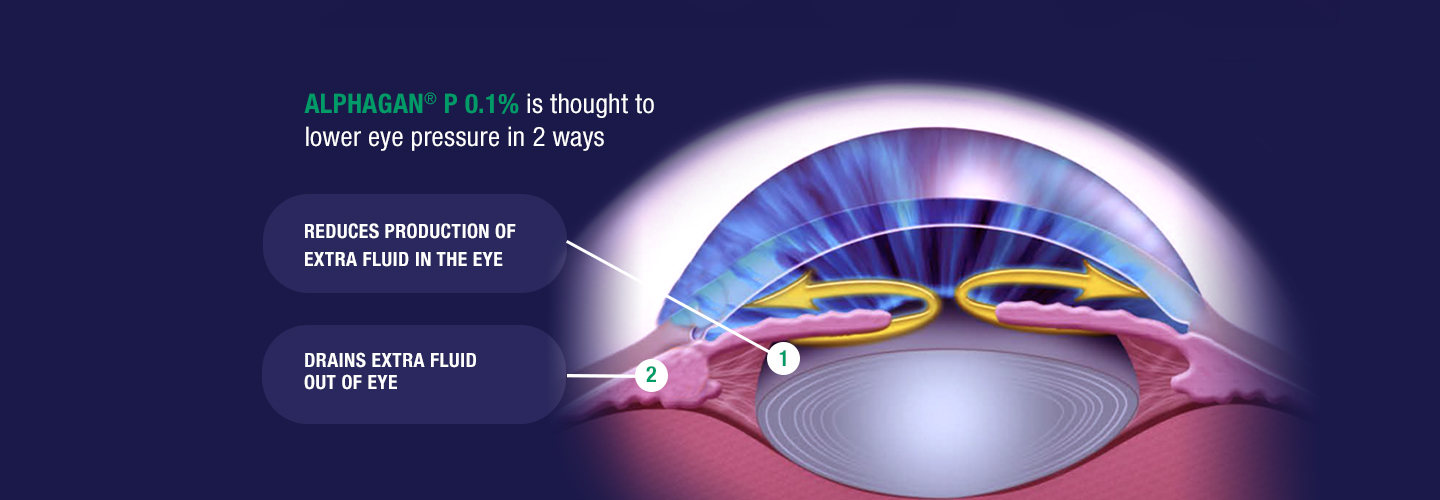
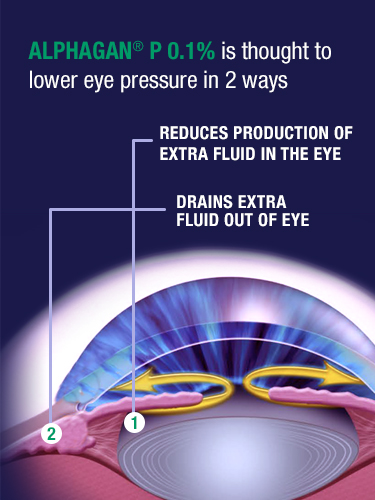
How is ALPHAGAN® P 0.1% thought to work?
Although the exact way ALPHAGAN® P 0.1% may work is unknown, it is thought to reduce eye pressure in 2 ways.
Be Sure You Get The
Green Bottle
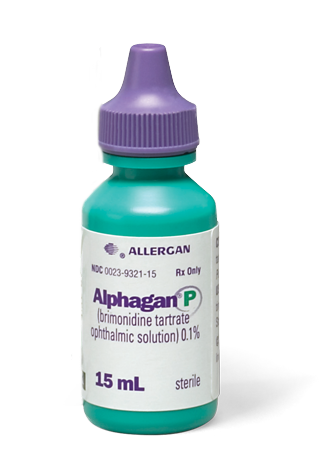
- Be sure to get ALPHAGAN® P 0.1% in the green bottle from your pharmacy. ALPHAGAN® P 0.1%:
- Is preserved with Purite®, which breaks down into natural tear components in the eye
- Is a proven, FDA-approved medication used 3 times a day about 8 hours apart, every day, to lower your high eye pressure
USE
ALPHAGAN® P (brimonidine tartrate ophthalmic solution) 0.1% or 0.15% is used for the reduction of high eye pressure, also called intraocular pressure (IOP), in patients with open-angle glaucoma or ocular hypertension.
IMPORTANT SAFETY INFORMATION
Do not use ALPHAGAN® P 0.1% and 0.15%:
- If you are allergic to any of the ingredients
- In neonates and infants (under the age of 2 years)
ALPHAGAN® P 0.1% and 0.15% may make syndromes associated with vascular insufficiency (conditions that result in reduced blood flow) worse.
ALPHAGAN® P 0.1% and 0.15% should be used with caution in patients with:
- Depression
- Cerebral or coronary insufficiency (conditions that result in reduced blood supply to the brain or heart)
- Raynaud’s phenomenon (a condition that results in reduced blood supply, most commonly, to the fingers and toes)
- Orthostatic hypotension (a form of low blood pressure that occurs when standing after sitting or lying down)
- Thromboangiitis obliterans (Buerger’s disease) (a condition that results in reduced blood supply to the arms and legs, usually presenting in fingers and feet)
- Severe cardiovascular disease (a range of conditions affecting the heart)
Avoid allowing the tip of the dispensing bottle to touch the eye, anything around the eye, fingers, or any other surface to avoid contamination by common bacteria known to cause eye infections (bacterial keratitis). Using contaminated solutions can cause serious damage to the eye and loss of vision. Always replace the cap after using. Do not use after the expiration date marked on the bottle or if the solution changes color or becomes cloudy.
If you have eye surgery, eye trauma or infection, or develop any eye reactions, immediately consult with your physician about continuing the use of ALPHAGAN® P 0.1% and 0.15%.
If you use more than one drug in the eye, be sure to wait at least 5 minutes between each drug application.
As with other similar medications, ALPHAGAN® P 0.1% and 0.15% may cause fatigue and/or drowsiness in some patients. Patients who engage in hazardous activities should be aware of the potential for a decrease in mental alertness.
The most common side effects (10%-20%) in the clinical trial include allergic eye reaction, eye redness, and itchy eye. Other side effects (5%-9%) include eye burning sensation, inflammation of the conjunctiva (“pink eye”), high blood pressure, dry mouth, and visual disturbance. These are not all of the possible side effects with ALPHAGAN® P 0.1% and 0.15%. Tell your doctor if you have any side effect that bothers you or that does not go away.
Please see full Prescribing Information for ALPHAGAN® P at https://www.rxabbvie.com/pdf/
alphagan_pi.pdf.
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.fda.gov/medwatch or call
1-800-FDA-1088.
If you are having difficulty paying for your medicine, AbbVie may be able to help. Visit AbbVie.com/myAbbVieAssist to learn more.



